
From the periodic table the molar masses of the compounds will be extracted. To convert grams to moles, the molecular weight of the solute is needed. The number of moles represents the fraction: mass of the compound / molecular weight of the compound. Here are the conversions: 1 mol/L (M) equals to Unit of measurement : SI: The concentration may also be expressed in different fractions of the molar concentration such as mmol/L (mM), μmol/L (μM), nmol/L (nM), pmol/L (pM). It is defined as the number of moles in a solution. This is the same with molar concentration and represents the concentration of a solute in a solution. Concentration – expressed in either molar, milimolar, micromolar, nanomolar, picomolar or femtomolar.Mass – expressed in either grams, milligrams, micrograms Whats the mass of NaCl required to make a 0.2mol/L 15ml NaCl solution in water The molar mass of NaCl is 58.44276 g/mol.Volume – expressed in either liters, milliliters or microliters.To compute any of the elements in the molarity equation you need to input the other three and choose the desired measurement unit.
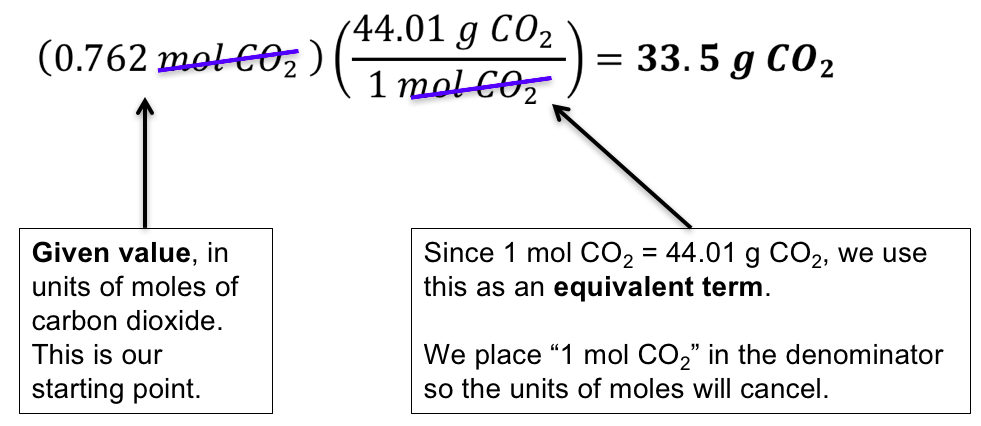
There are four components in the equation therefore there are four fields in the molarity calculator. There are three elements - carbon, hydrogen and oxygen - so find their atomic masses in the periodic table: 12.0107 grams per mol for carbon, 1.00794 grams per. This tells you that you get #"25.7 g"# of rubidium nitrate for every #"100 g"# of the solution.This is a great chemistry tool for those who need to determine a solution’s molar concentration from the mass, volume and molecular weight. This means that the solution's percent concentration by mass is equal to So, you know that you have #"346.56 g"# of rubidium nitrate in #"1346.56 g"# of the solution, so you can say that #"100 g"# of this solution will contain #"346.56 g " + quad 10^3 quad "g" = "1346.56 g"# Molar concentration is defined as the amount of a constituent (usually measured in moles ) divided by the volume of the mixture. This means that the total mass of the sample is equal to

For example, if you want to find the molar mass of. We can find the molar mass of a compound using its. Use the molar mass of the solute to convert the number of moles to grams The molar mass of elements is found by looking at the atomic mass of the element on the periodic table. Part of our molar mass calculator and chemistry helper tools site. As we've said, this sample will also contain #2.35# moles of rubidium nitrate. To make the calculations easier, pick a sample of this solution that contains exactly #"1 kg" = 10^3 quad "g"# of water. This tells you that this solution contains #2.35# moles of rubidium nitrate, the solute, for every #"1 kg"# of water, the solvent. Now, you know that the solution has a molality equal to #"2.35 mol kg"^(-1)#. the solution's percent concentration by mass, #"% m/m"#.
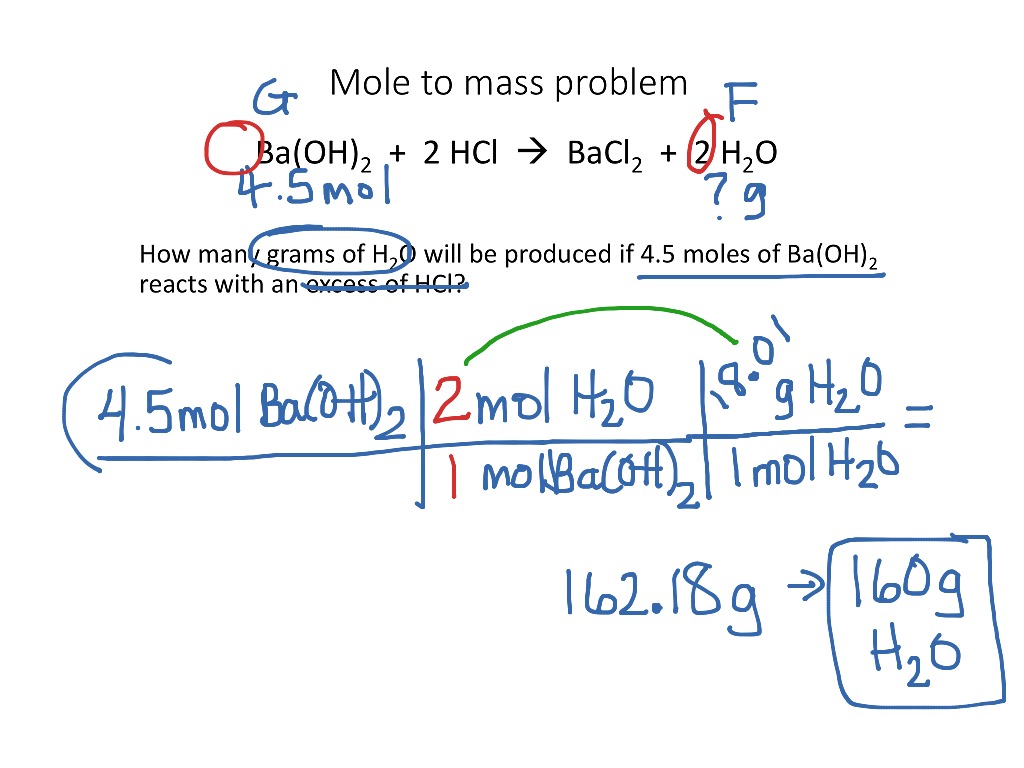
Your goal here is to figure out the number of grams of solute present for every #"100 g"# of the solution, i.e.


 0 kommentar(er)
0 kommentar(er)
